Researching how the pepper grows
Leibniz Institute of Plant Biochemistry (IPB) elucidates biosynthesis of pungency
Advertisement
How do plants produce pungent substances? The Leibniz Institute of Plant Biochemistry (IPB) is working intensively on this topic. Recently, scientists led by Dr. Thomas Vogt have found the decisive enzyme that helps the fruits of the pepper plant (lat. Piper nigrum) to achieve their characteristic pungency. The enzyme discovered, piperine synthase, catalyzes the final step in the biosynthesis of the pungent piperine. Now the biochemists have followed up with a second pungent compound and also shed more light on the biosynthesis of Capsaicin from the chili pepper (Capsicum spec.). For the first time, his research group describes the enzymatic activity of the long-sought capsaicin synthase. The enzyme catalyzes the final reaction step in plant capsaicin production.
Piperine and capsaicin bind on the tongue and mucous membranes to the very receptor that also responds to heat, acids or injury. This receptor triggers a stimulus in certain nerve cells that humans perceive as spiciness. Capsaicin and piperine also have antimicrobial effects as well as promoting digestion and blood circulation. They are therefore not only interesting as pungents, but also as active ingredients for medical applications. "You can find hundreds of studies on the biological effects of pepper and chili extracts in the literature of recent years, but very few on the biosynthesis of the pungent substances," explains Thomas Vogt, who heads the Phenylpropane Metabolism research group at IPB.
This may be due to the fact that the elucidation of plant biosynthetic pathways requires different expertise, which is only available in this combination at a few research institutes. Ideally, chemists, biochemists and bioinformaticians pull together to fish out of the hundreds of enzymes in a plant the handful of candidates that are involved in the production of a sought-after substance. This search for individual enzymes is particularly difficult in those plant species of which most genes and enzymes are still unknown. "Because here," says Thomas Vogt, "you can only consult the databases to a limited extent."
In the case of pepper, the data situation was also thin, and there were further hurdles. For example, the scientists from Halle first had to grow pepper plants and, above all, make sure they bore fruit. "That's not a given with pepper, which grows in a greenhouse," explains the biochemist, "but the gardeners at the institute succeeded." With successful cultivation of the plants, the research team was able to harvest pepper berries at various stages of ripeness over a period of three months, and here they were able to closely observe how their piperine content steadily increased. According to the scientists, the ripening berries should also contain the enzymes that form piperine. The leaves of the plant, on the other hand, should not contain the piperine biosynthesis enzymes, because they do not produce piperine and therefore do not taste spicy.
The scientists took advantage of these differences in piperine content, i.e. in the presence and absence of the piperine biosynthesis enzymes. Based on the premise that an enzyme is only present in certain tissues when its gene is activated and read, they compared gene activities in leaves and fruits of different maturity stages. This enabled them to identify those genes that are particularly active in the immature fruits. Among them was the gene for piperine synthase. This gene was then introduced into microorganisms and served as a template for the bacteria to produce the piperine synthase enzyme. The pepper experts from Halle were then able to prove beyond doubt that the isolated enzyme catalyzes the fusion of the two starting substances piperoyl-CoA and piperidine to form piperine. This was proof that this was indeed the piperine synthase they were looking for.
The discovery of capsaicin synthase posed other problems. Here, the coding gene had already been identified, but science had never before succeeded in having an enzymatically active protein produced from it in bacteria. Proof that the gene under investigation was capsaicin synthase at all was therefore still pending. But with their expertise from the relatively similar pepper enzyme, the plant researchers from Halle were able to isolate capsaicin synthase and prove in the final activity test that it does indeed catalyze the long-postulated reaction of the two starting substances, 8-methyl-6-nonenoyl-CoA and vanilloylamine, to capsaicin. This final proof of activity for piperine and capsaicin synthase was achieved partly because the institute's synthesis chemists were able to produce all the starting materials themselves, which are not yet available for purchase, and make them available for the enzyme tests.
The most important reaction steps of piperine biosynthesis and capsaicin biosynthesis have thus been elucidated. At some point, both biosynthetic pathways will be fully understood. But what is the benefit of this knowledge? With the knowledge of all the enzymes involved in a biosynthesis, one can introduce the corresponding genes into microorganisms and have them produce the desired active ingredient. "In the case of pepper, however, this would not be economical," says Thomas Vogt, "because piperine is present in pepper berries in such high concentrations that it could be isolated very easily, for example for medicinal purposes."
For the scientists at IPB, the elucidation of biosynthetic pathways is nevertheless worthwhile, because the newly discovered enzymes catalyze interesting reactions that lead to reaction products with complicated structures. These enzymes can be modified by biochemists to produce completely new enzymes with desired properties. The optimized enzymes are then used to design syntheses of new, potentially active substances in the test tube. This type of biocatalytic synthesis - i.e. the imitation and optimization of the original plant biosynthesis in the test tube - has enormous future potential as a relatively young field of research. As an alternative to petrochemical syntheses, biocatalysis can produce desired substances without the use of toxic catalysts and solvents or the generation of harmful byproducts.
Background: biosyntheses and enzymes
Enzymes are proteins found in every living thing and in every cell. Every reaction in the body that converts one substance into another (metabolism) is catalyzed by an enzyme. There are thousands of metabolic reactions in every organism and correspondingly just as many different enzymes. In this context, several successive individual reactions form separate metabolic or biosynthetic pathways that lead to many different end products. In their ability to produce a wide range of complex substances, plants are superior to animals. This is why they have many more enzymes than animal organisms. Humans have been using enzymes for thousands of years, for example to produce bread, sauerkraut, alcohol and cheese.
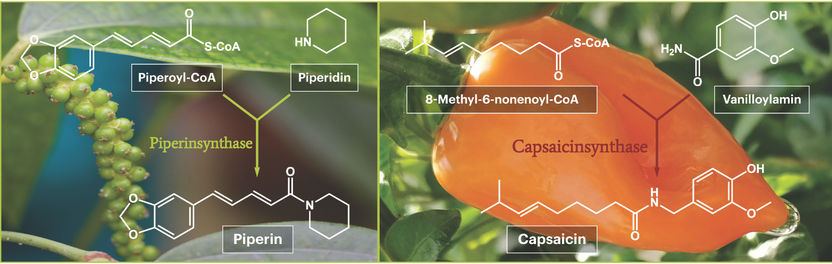
The final biosynthesis step of pungent piperine from the pepper plant (left) and capsaicin production in hot chili fruits (right).
Thomas Vogt, IPB
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.





























































